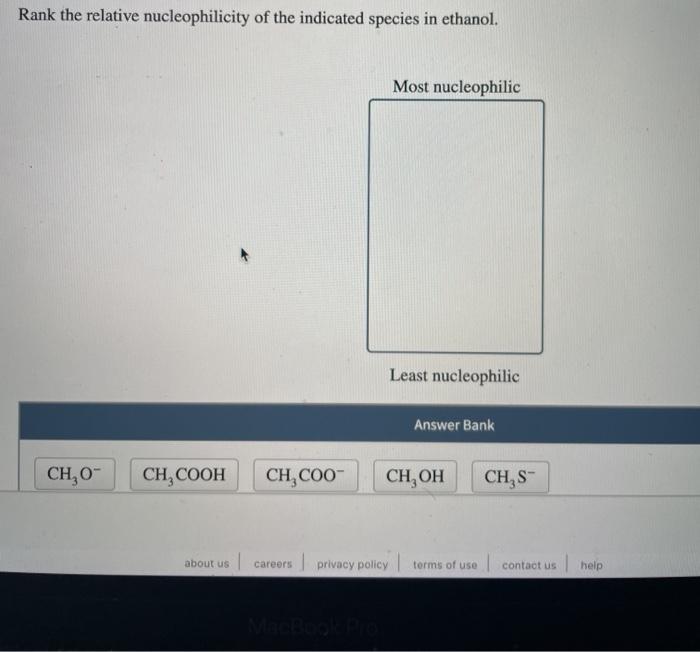
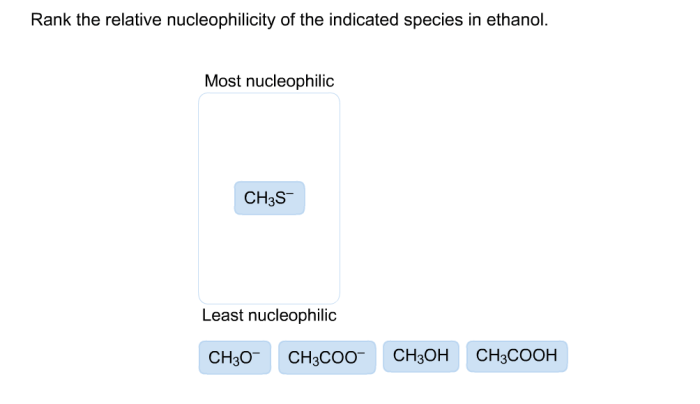
Rank the relative nucleophilicity of the indicated species in water, a topic of fundamental importance in chemistry, unveils the intriguing interplay between electronic structure and reactivity. Nucleophilicity, a measure of an atom’s or molecule’s ability to donate electrons, profoundly influences the course of chemical reactions.
Water, with its unique properties as a solvent, presents a fascinating medium for exploring nucleophilicity and its consequences.
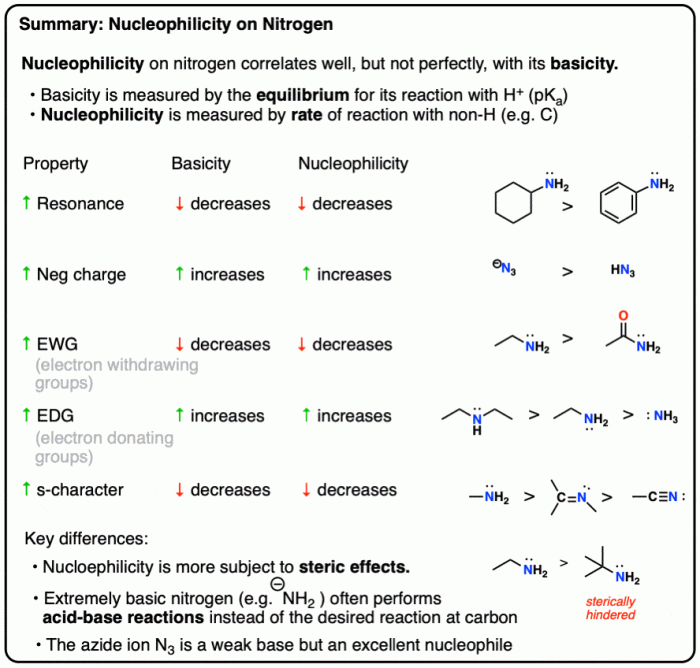
Factors such as charge, hybridization, and resonance exert a profound influence on nucleophilicity. This article delves into these factors, elucidating their impact on the nucleophilic behavior of various species. Armed with this knowledge, chemists can harness nucleophilicity to predict reaction outcomes and design effective synthetic strategies.
Factors Affecting Nucleophilicity in Water

The nucleophilicity of a species in water is influenced by both electronic and steric factors. Electronic factors include the charge, hybridization, and resonance of the nucleophile. Steric factors include the size and shape of the nucleophile.
Electronic Factors, Rank the relative nucleophilicity of the indicated species in water
The charge of a nucleophile has a significant effect on its nucleophilicity. Nucleophiles with a negative charge are generally more nucleophilic than neutral nucleophiles, which are in turn more nucleophilic than positively charged nucleophiles. This is because the negative charge on a nucleophile makes it more attractive to electrophiles.
The hybridization of a nucleophile also affects its nucleophilicity. Nucleophiles with sp 3hybridization are generally more nucleophilic than nucleophiles with sp 2hybridization, which are in turn more nucleophilic than nucleophiles with sp hybridization. This is because the sp 3hybridization results in a more diffuse electron cloud, which makes the nucleophile more accessible to electrophiles.
Resonance can also affect the nucleophilicity of a species. Nucleophiles that can resonate are generally more nucleophilic than nucleophiles that cannot resonate. This is because resonance stabilizes the negative charge on the nucleophile, making it more attractive to electrophiles.
Steric Factors
The size and shape of a nucleophile can also affect its nucleophilicity. Nucleophiles that are small and unhindered are generally more nucleophilic than nucleophiles that are large and hindered. This is because the small and unhindered nucleophiles can more easily reach the electrophile.
FAQ Insights: Rank The Relative Nucleophilicity Of The Indicated Species In Water
What factors influence nucleophilicity in water?
Charge, hybridization, and resonance are key factors that affect nucleophilicity in water.
How can nucleophilicity ranking be applied in organic chemistry?
Nucleophilicity ranking aids in predicting reaction outcomes and designing synthetic strategies in organic chemistry.
What is the significance of water as a solvent for nucleophilic reactions?
Water’s unique properties, such as its polarity and ability to form hydrogen bonds, influence the nucleophilicity of species dissolved in it.