Introducing the formula for a hydrate lab answers, a comprehensive guide that delves into the fascinating realm of hydrates. From understanding their unique chemical composition to exploring their diverse applications and environmental implications, this exploration unveils the secrets of these captivating compounds.
Hydrates, captivating substances that entrap water molecules within their crystalline structures, play a pivotal role in various scientific disciplines and industrial processes. This detailed analysis delves into the experimental techniques employed to determine their formulas, highlighting the significance of accurate measurements and calculations in ensuring reliable results.
Formula for a Hydrate
A hydrate is a compound that contains water molecules loosely bound to the central metal ion or molecule. The chemical formula of a hydrate is written as: $$M_x(H_2O)_y$$ where M represents the metal or molecule, x is the number of metal ions or molecules, and y is the number of water molecules associated with each metal ion or molecule.
Common examples of hydrates include:
- Copper(II) sulfate pentahydrate, CuSO 4·5H 2O
- Sodium chloride dihydrate, NaCl·2H 2O
- Calcium chloride hexahydrate, CaCl 2·6H 2O
The water molecules in a hydrate are held in place by hydrogen bonds. These hydrogen bonds are relatively weak, so the water molecules can be easily removed by heating or exposing the hydrate to a dry atmosphere.
Lab Analysis of Hydrates

The formula of a hydrate can be determined by a variety of methods, including gravimetric analysis and Karl Fischer titration.
Gravimetric analysisinvolves weighing the hydrate before and after it has been heated to remove the water molecules. The difference in weight is equal to the mass of the water molecules, which can then be used to calculate the number of water molecules per metal ion or molecule.
Karl Fischer titrationis a volumetric method that involves reacting the water molecules in the hydrate with a known amount of iodine. The amount of iodine that is consumed is equivalent to the number of water molecules in the hydrate, which can then be used to calculate the formula of the hydrate.
Applications of Hydrates: Formula For A Hydrate Lab Answers

Hydrates have a wide variety of applications in different industries, including:
- Pharmaceuticals:Hydrates are used as excipients in many pharmaceutical formulations. They can help to improve the stability, solubility, and bioavailability of drugs.
- Food preservation:Hydrates are used as preservatives in a variety of foods, including fruits, vegetables, and meat. They help to prevent spoilage by inhibiting the growth of bacteria and mold.
- Construction:Hydrates are used in a variety of construction materials, including cement and plaster. They help to improve the strength and durability of these materials.
Hydrates also have some disadvantages, including:
- Efflorescence:Hydrates can lose water molecules when exposed to a dry atmosphere, which can cause them to crumble or disintegrate.
- Deliquescence:Hydrates can absorb water molecules from the air, which can cause them to become wet or sticky.
Environmental Impact of Hydrates

Hydrates can have a significant impact on the environment, particularly in the context of climate change.
Carbon sequestration:Hydrates can store large amounts of carbon dioxide. This makes them a potential target for carbon capture and storage (CCS) technologies, which aim to reduce greenhouse gas emissions.
Methane release:Hydrates can also release methane, a potent greenhouse gas. This can occur when hydrates are exposed to warmer temperatures, such as those that are expected to occur as a result of climate change.
To minimize the environmental impact of hydrate-related activities, it is important to carefully consider the potential risks and benefits of these technologies.
FAQ Section
What is the general formula for a hydrate?
The general formula for a hydrate is M(H2O)n, where M represents the metal cation and n is the number of water molecules coordinated to the metal ion.
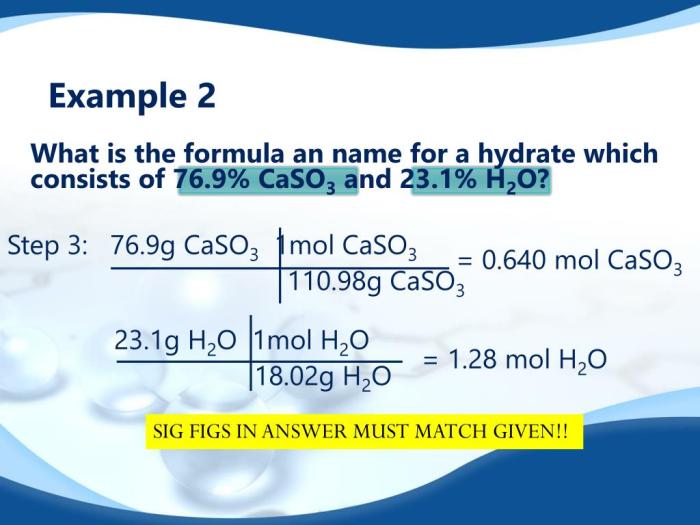
How is the formula of a hydrate determined in the lab?
The formula of a hydrate can be determined using gravimetric analysis or Karl Fischer titration. Gravimetric analysis involves measuring the mass of the water lost when the hydrate is heated, while Karl Fischer titration involves reacting the water in the hydrate with a Karl Fischer reagent.
What are some applications of hydrates?
Hydrates have a wide range of applications, including in pharmaceuticals, food preservation, and construction. In pharmaceuticals, hydrates are used to control the release of drugs in the body. In food preservation, hydrates are used to prevent spoilage. In construction, hydrates are used to make concrete and other building materials.